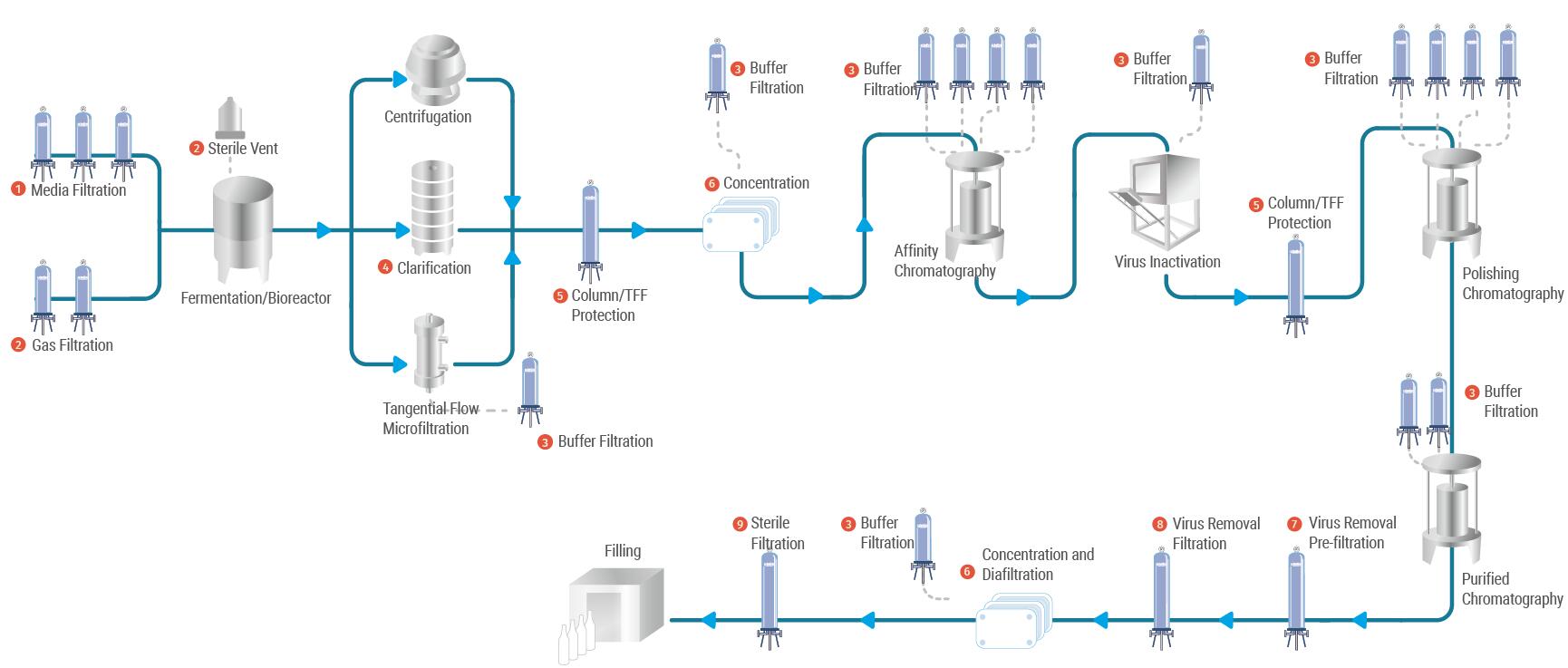
Membrane filtration is an indispensable step in the production of antibody drugs. It is used in a wide range of applications such as sterilization of culture media and buffers, rapid separation of cells from supernatant after culture media has been discharged from the tank and gas filtration in fermentation processes. For the production of antibodies, combinations of efficient and fast membrane filtration products, TFF systems and safe & reliable disposable solutions are available from Cobetter.
Antibody is a Y-shaped protein produced by the body to stimulate effector B cells due to the invasion of foreign substances such as bacteria and viruses, etc. It consists of two heavy chains and two light chains, which are used by the immune system to identify and neutralize foreign substances. It also consists of two heavy chains and two light chains, which are used by the immune system to identify and neutralize foreign substances and are only found in body fluids such as blood of vertebrates and on the surface of the cell membrane of B cells.
The monoclonal antibody technique was invented in 1975 by British scientists Milstein and Kohler, and won the 1984 Nobel Prize in Medicine. The development of monoclonal antibody drugs is only 45 years old and has become a global driver of biologic drug development due to its efficiency, targeting and low side effects. Seven of the top ten drugs sold worldwide are antibody drugs, with Chinese antibody drug sales reaching RMB 18 billion in 2019 and expected to reach RMB 30 billion in 2020.
The monoclonal antibody drug process has also undergone rapid development in cell culture technology and downstream purification technology. In particular, upstream cell culture has developed from tens of mg/litre in the 1980s to more than 5g/litre today, which has greatly reduced the cost of cell culture. Downstream purification and capture stage of Protein A affinity packing not only greatly increased the load and flow rate, packing also developed from not resistant to alkali cleaning, to today can withstand 1mol NaOH. At the same time, in order to improve production efficiency, to ensure safety, pollution-free, disposable products are widely used in the production of many antibody processes.
|